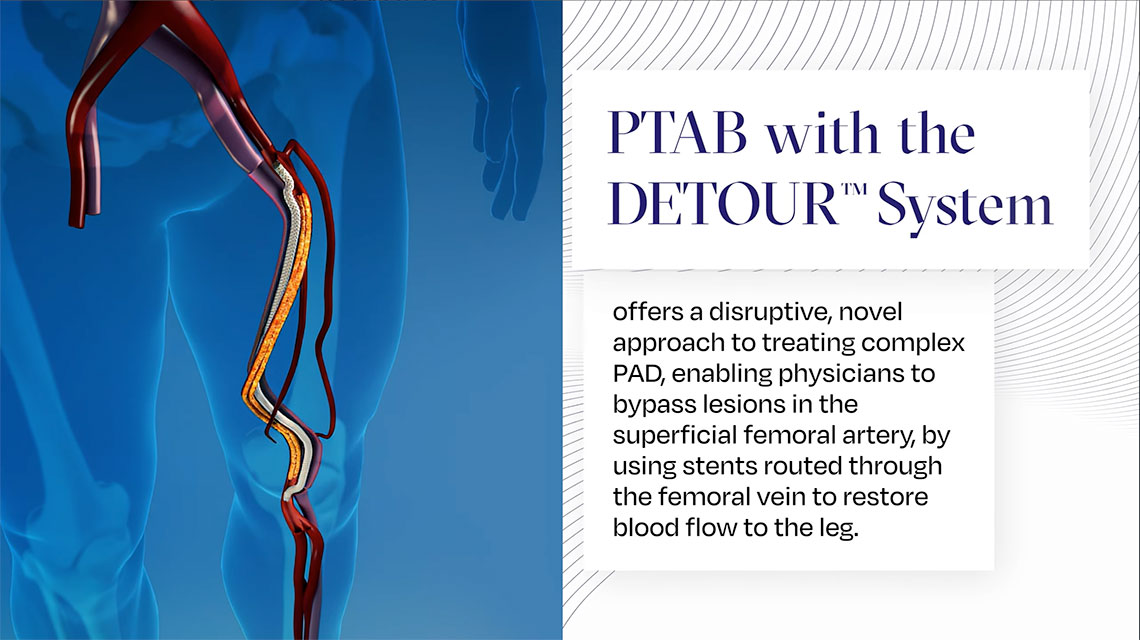
Endologix receives FDA Approval of the DETOUR™ System to Treat Long Complex Superficial Femoropopliteal Lesions in Patients with PAD.
Endologix LLC, a privately held, global medical device company dedicated to providing disruptive therapies for the interventional treatment of vascular disease, announced today that the U.S. Food and Drug Administration (FDA) has granted approval for the DETOUR System to treat patients with complex peripheral arterial disease (PAD).
Over 8.5 million Americans are affected by PAD. This condition can cause significant pain, reduced mobility, and even amputation. At present, patients with long blockages of the superficial femoral artery (SFA) have limited treatment options. The recommended therapy is open surgical bypass, which is invasive, has a high early complication rate and requires prolonged recovery. Endovascular treatments are feasible in selected patients but have limited patency.
Read more…



